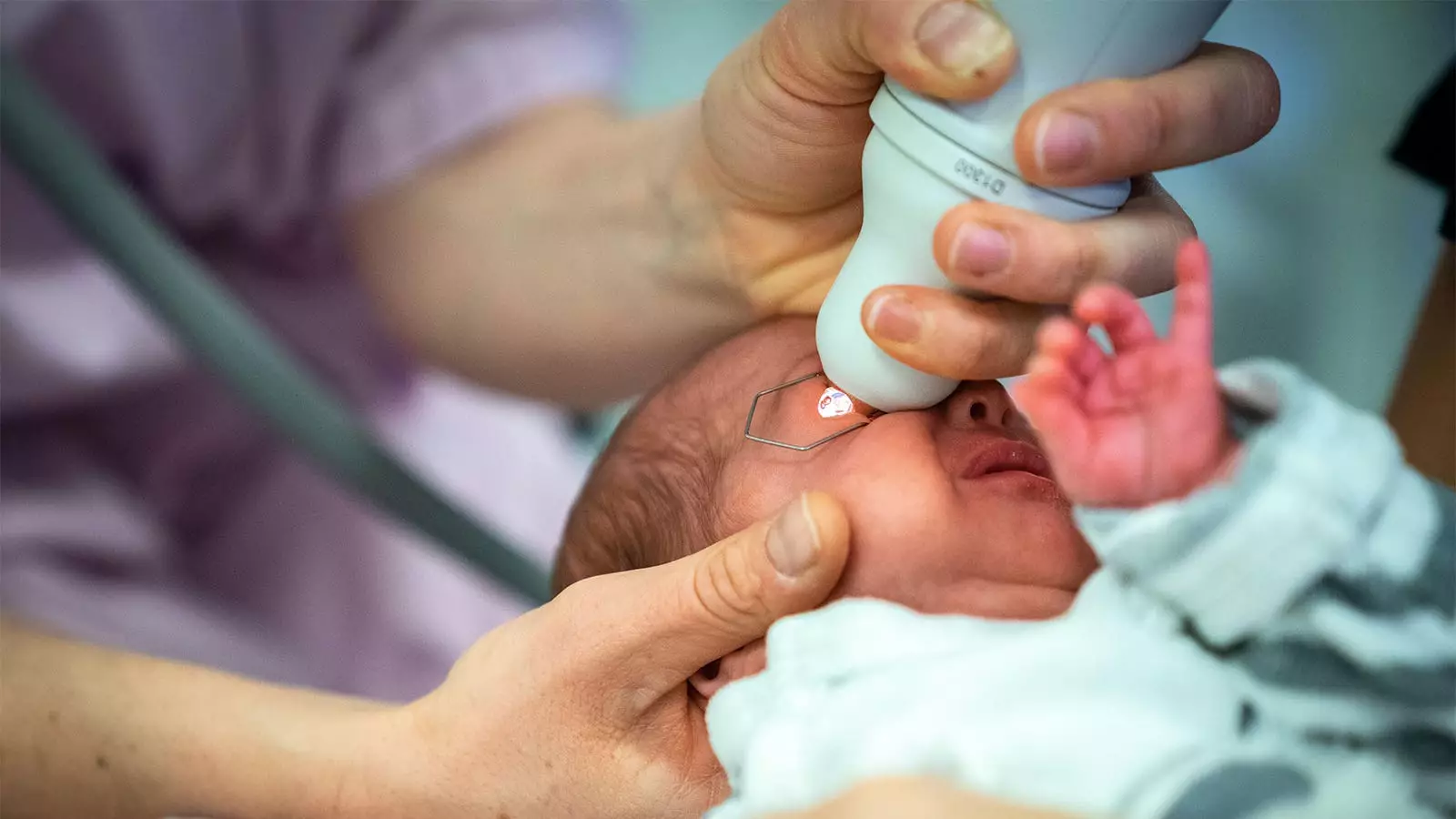
Retinopathy of Prematurity (ROP) is a critical concern for preterm infants, often requiring numerous eye examinations that can be invasive and potentially harmful. A recent study highlights the potential of mydriatic microdrops as a safer alternative to conventional mydriatic drops, striving to mitigate the associated risks while ensuring effective screening. This article will break down the findings of this pivotal research, explore its implications for clinical practice, and highlight areas for further study.
A randomized trial conducted by Dr. Asimina Mataftsi and her research team from Aristotle University of Thessaloniki assessed the effectiveness and safety of microdrops compared to standard mydriatic drops in a cohort of 83 preterm infants. Previous anecdotal reports suggested adverse effects accompanying ROP screening, prompting the need for this rigorous analysis. The primary focus was on whether these microdrops could induce sufficient mydriasis, or pupil dilation, without the systemic risks attributed to larger doses of traditional drops.
The trial involved infants with gestational ages below 32 weeks or birth weights under 1,501 grams, further emphasizing the vulnerability of this population. Its findings demonstrated not only the noninferiority of microdrops at longer intervals post-administration but also superior efficacy at the first measurement point at 45 minutes. At this juncture, the mean difference in dilation was statistically significant, allowing for the possibility that microdrops could transform standard care practices.
Mydriatic drops traditionally employed during ROP screening have been linked to various adverse cardiovascular, gastrointestinal, and respiratory effects. Given the unique physiological circumstances of preterm infants — including extremely low body mass and immature organ systems — these outcomes can be particularly severe. The study noted a concerning trend in lower oxygen saturation levels and a higher incidence of hypertensive episodes following the application of standard drops. These findings underscore the need to reconsider the existing protocols for administering mydriatics in this sensitive demographic.
Moreover, anecdotal data from neonatal intensive care unit (NICU) personnel further elucidates the risks associated with ROP screenings. Reports of increased apnea events and general deterioration in infants after examinations heighten concerns regarding the broader implications of using traditional mydriatics.
The outcomes of the MyMiROPS study suggest a promising avenue for improving the safety of ROP screening. The microdrops demonstrated sufficient efficacy in achieving necessary pupil dilation while significantly reducing the risk of adverse systemic reactions. The study’s findings mark an important advancement in the care of preterm infants, suggesting that this method can enhance clinical practices.
Dr. Mataftsi and her colleagues also pointed out striking similarities in peripheral blood levels between microdrops and standard drops, despite the latter’s greater volume. Such findings posit that smaller doses may provide sufficient systemic exposure to pharmacological agents without the trade-off of increased side effects, leading to a potentially safer standard of care in neonatal settings.
Yet, important limitations exist within the study’s design. Notably, safety outcome data was absent for around 30% of cases, necessitating caution in fully endorsing microdrops without additional validation through larger and more comprehensive trials. Future research is crucial to fully understand the health impacts of mydriatics and to explore potential benefits across different infant populations and regimens.
The exploration of mydriatic microdrops for ROP screening represents a significant shift in neonatal ophthalmology. As efforts continue to balance clinical efficacy with patient safety, this study lays the groundwork for future innovations in eye care for the most vulnerable infants. Ultimately, the pursuit of improved ROP screening techniques that prioritize infant safety will be paramount. With further studies to corroborate these findings, we may soon witness a paradigm shift that redefines best practices and enhances outcomes for preterm infants at risk of ROP.

Leave a Reply